Here is a lab we did in science class to see if the density of minerals depend on the size of them
“Does the density of a mineral depend on the size of the mineral sample?”
Hypothesis:
“I think that if you take a big mineral it is more likely to be less dense than a smaller mineral of the same kind because the small one is more compact.”
Controlled Variable:
The amount of water in the graduated cylinder that you put the mineral in is the controlled variable.
Manipulated Variable:
The manipulated variable is the size of a mineral.
Responding Variable:
The responding variable is the mass, weight, and the density.
Materials:
In the lab we used a balance scale, Pyrite, Galena, and a graduated cylinder.
Procedure:
Procedure:
1. Get all of your materials.
2. Get two big and small minerals of each.
3. Weigh them all one at a time and copy them down.
4. Put one into a graduated cylinder and then see how much the water rose, copy that down and do the same for each of them.
5. Now you have the mass(weight) and volume, you divide mass by volume: Mass/Volume.
6. The answer to that problem is the density, so now you get the average for the big and small minerals and you have the answer to the lab. (If the number is higher it is denser)
Record and analyze:
While we were doing this experiment I noticed that we needed a smaller graduated cylinder because with the small minerals the other one was too big to see a difference. Also I saw that the small minerals have smaller volume and mass so when you divide them they should be the same as the bigger minerals. Another thing that I noticed while doing the tests is that when they are heavy that means that they are denser.
Data Table:
| |||||||||||||||||||||||||||||||||
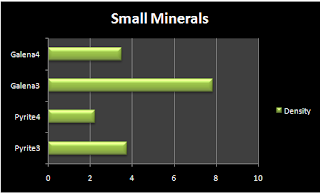
Small Minerals | |||
Mineral | Mass | Volume | Density |
Pyrite3 | 15g | 4 ml | 3.75 |
Pyrite4 | 8.9g | 2 ml | 2.225 |
Galena3 | 47g | 6 ml | 7.83 |
Galena4 | 14g | 4 ml | 3.5 |
Average | Density 4.32625 | ||
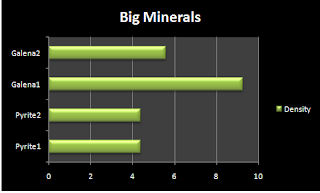
Graph:
Data Analysis:
From the graphs I can tell that the densities do not have too much of a difference to say that there is a certain size denser than the other size. From the results I would say that the size does not matter. I researched the actual data and some of the answers we got were not so far away from the correct answer.
Conclusion:
Now from the tests we did I can see that my hypothesis was incorrect. The density does not rely on the size of a mineral. I thought about how you come up with mass divided by volume, and I think the answer is quite simple. I think you take the volume so you know how much there is of the object, then you get the mass to see how much weight there is and when you divide it you get density because you know the space in it so the mass tells you how much is in the space given so when you divide them you get density. I also realized that the little difference does not change too much, if the number are close enough it does not make too much of a difference.
Further Inquiry :
I think in this project I did well. However I know there are many ways to improve. For example, we could have used a few more minerals to get more accurate data. The actual density of Pyrite was close in the big ones but the small ones were quite of-- it is actually 5.01. Before I made the conclusion I wanted to know the actual density so I can give a correct conclusion. The correct density for Galena is 7.6. Some of the answers I got were a bit close to it, and I would say that when you are not that old and do not have such high-tech materials that we did well.
No comments:
Post a Comment